
The Role of Remodeling of the Extracellular Matrix (ECM) in Breast Cancer Progression: The mammary ECM is made up of a complex mesh of proteins, primarily collagen, which is maintained by fibroblast cells. These fibroblast cells create and breakdown tissue as needed, and apply tension on the mesh to protect other cells. Fibroblasts also use the ECM to send mechanical cues to mammary epithelial cells (MECs) in order to maintain a normal equilibrium, or homeostasis. In cancer, this homeostasis is no longer maintained. We predict that these mechanical cues play a significant role in the growth of breast cancer. We found evidence of this in MEC cultures created with and without fibroblasts, where the size and shape of MEC organoids were influenced by fibroblast density. Our laboratory has previously investigated methods of determining the elastic properties of breast tissue by monitoring mechanical waves as the propagate through tissue phantoms (Li et al, 2011; Mohan et al, 2012). Recently, we have developed Diffusion-Sensitive Optical Coherence Tomography (DS-OCT) to explore the nanoporosity of tissue (Blackmon et al, 2016).
DS-OCT is a minimally-invasive imaging technique that measures the rate at which GNRs diffuse through live tissue. GNRs are particularly well suited to study the ECM due to their unique polarization-dependent optical properties. The diffusion rate of the GNRs is sensitive to both viscosity of liquids (Chhetri et al, 2013) and nanoporosity of tissue, such as collagen density (Blackmon et al, 2016) or mucus concentration (Chhetri et al, 2014). Therefore, GNR diffusion can be used as a measure of ECM remodeling by sensing changes in nanoporosity. This is shown in Figure 1(a), where the rate of GNR diffusion decreases as the pore sizes of collagen decrease at higher concentrations.
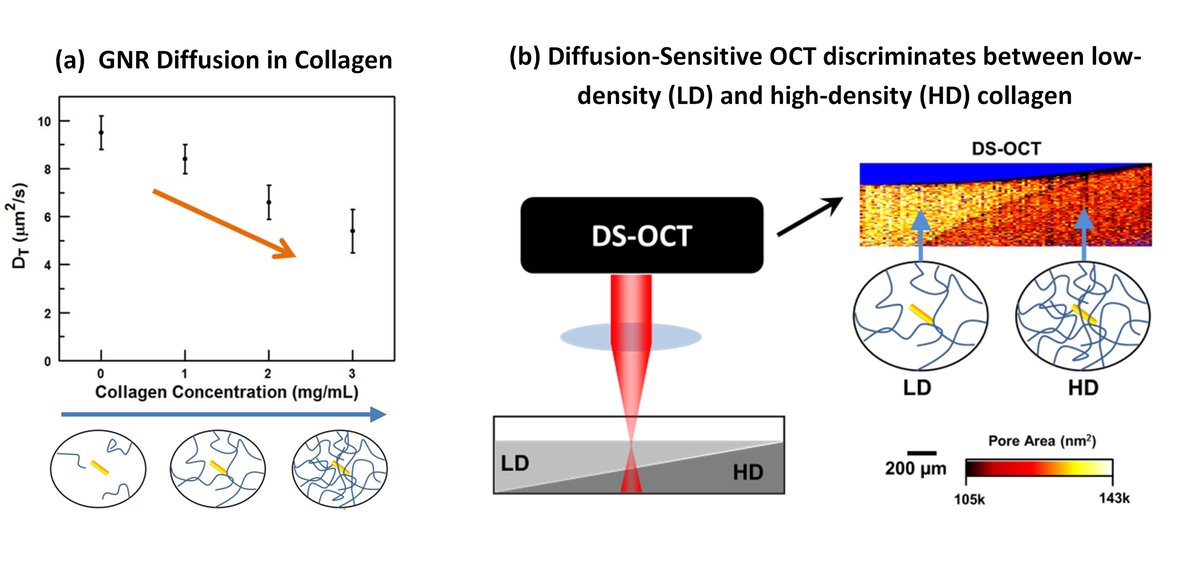
Fig. 1. (a) Increases in the concentration of collagen results in more constrained GNRs, which translates to a slow GNR diffusion rate. This measured diffusion rate therefore provides a method of quantifying the pore areas of the collagen. (b) DS-OCT was used to measure pore areas in a sample with layered collagen, with low-density (LD) collagen layered on top of high-density (HD) collagen.
The anomalous increase in GNR diffusion in cell cultures with high seed densities motivated us to develop a technique to spatially resolve GNR diffusion rates. DS-OCT (link to DS-OCT method) measurements are spatially resolved, giving us the ability to sense differences in pore sizes in volumes as small as 470 um3. We first tested whether or not DS-OCT could spatially resolve nanoporosity in layered collagen samples (Figure 1(b)). Since we knew to expect small pore in the high-density collagen and large pore in low-density collagen, we were able to validate that DS-OCT was providing accurate results.
With DS-OCT validated, we measured pore areas of artificial ECM, with one containing no cells, one with a seed density of 50 cells/uL, and one with 100 cells/uL seed density, as reported in Blackmon et al, 2016. Shown in Figure 2, DS-OCT revealed the ECM remodelling that is undetectable with the intensity-based cross-polarized OCT images, including decreased overall pore sizes for increasing number of cells, damaged ECM (black arrows), and cell clusters (blue arrow). Conventional histology is included for comparison, which reveals locations of cells (red arrows), but does not provide information on pore sizes of the tissue. Importantly, DS-OCT was performed on these cell cultures while they were alive, and with no damage. DS-OCT thus provides a valuable new tool to study the role of ECM mechanics in breast cancer progression and drug delivery.
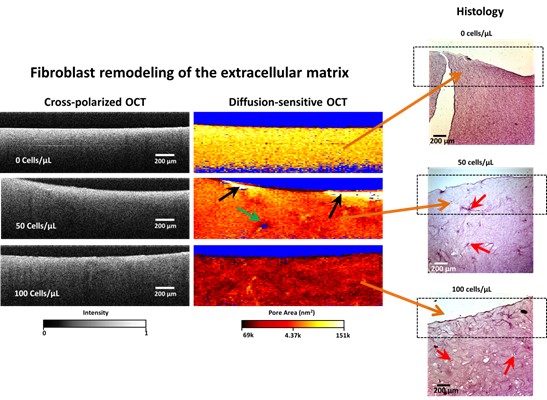
Fig. 2. DS-OCT reveals fibroblast remodeling of ECM by RMFs in 3D cell culture, that is validated with histology. This remodeling is detectable in conventional intensity-bassed cross-polarized OCT. Black arrows: Evidence of ECM degradation. Green arrow: evidence of a cell cluster due to the lack of a DS-OCT signal (blue pixels), which provides evidence that GNRs are not penetrating the cell membrane. Red arrows: locations of cell clusters in the histology slices.
intro page - research - publications - people - open positions
UNC Physics & Astronomy - Biomedical Research Imaging Center - UNC Home