"Polycyclic aromatic hydrocarbon" is just a mouthful for an organic molecule in space—not just any organic molecule, but one with a structure sturdy enough to survive the harsh environment between stars. Usually called "PAHs," this class of molecule is almost cerntainly the culprit responsible for the unidentified infrared emission (UIR) bands discovered in the early 1970s. The UIR bands are seen in many different kinds of objects from starburst galaxies to comets, so understanding their origin is very important. Because the UIR bands are so common, the PAHs which produce them must account for a sizeable fraction of all interstellar carbon in our galaxy and others. Estimates run as high as 15% or more!
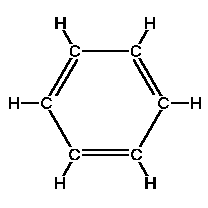
The place to start is with the simplest PAH: benzene. It's a hydrocarbon because it consists entirely of hydrogen and carbon (C6H6). The carbon atoms are arranged in a hexagonal structure with one hydrogen atom attached to each carbon atom. Because benzene has only one ring, it's not really polycyclic (does this make it monocyclic—a MAH?).
Each carbon atom has four electrons to share. One goes to the hydrogen atom, and one each to the two neighboring carbons. This leaves one to share with one of its two neighboring carbon atoms, which is why the benzene molecule is drawn with alternating single and double bonds around the hexagon. But at any time, there's no way to know which bonds are single and which are double. Many chemists just draw a circle around the inside of the ring to show that there are six electrons floating around. Hydrocarbons with this structure are called aromatic (because they generally stink—no kidding!) The electrons float above and below the ring, and the electromagnetic fields they generate keep the ring flat.
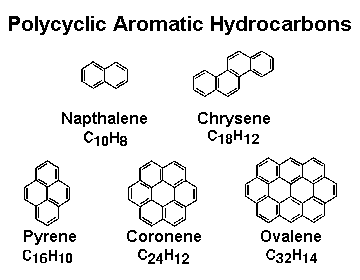
PAHs generally consist of several hexagonal rings. The figure to the left illustrates a few common simple PAHs. This figure was adapted from a figure produced by the NASA Ames Astrochemistry Lab., whose home page contains lots more information about PAHs and how they're studied on Earth. My focus is on PAHs in space.
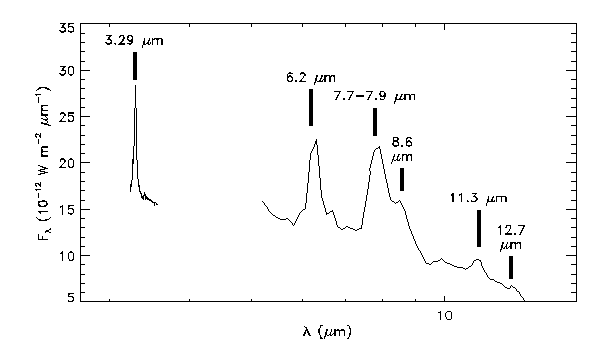
Above: A spectrum of the Red Rectangle, showing the Unidentified Infrared Emission Bands from 3 to 13 µm. These bands appear in the infrared spectra of many objects, and remained an enigma for over a decade after their discovery. The 3 µm data are from Sloan et al. (1994), the 5-8 µm data were provided by Jesse Bregman, and the 7-14 µm data are from Sloan et al. (1993).
Kris Sellgren, while working on her Ph.D. at Caltech, made a discovery that turned out to be the key in unravelling the mystery. She found that reflection nebulae which were sources of the UIR spectrum also emitted a continuum spectrum in the near-infrared from dust grains heated to 1000 K. This temperature was too hot to be from grains in equilibrium, so Sellgren hypothesized that while most of the grains were far cooler than this, some would be heated for very short periods of time to 1000 K by the absorption of a UV photon and would then quickly cool to much lower temperatures. The hot grains, while only a small fraction of the total grain population, would dominate the near-infrared emission.
The most likely suspect for the UIR bands had to be a carbon-based dust grain, because no other material would be able to withstand the harsh UV environment where the UIR bands were seen. Sellgren's discovery meant that the grains had to be extremely small, because larger grains would not change their temperature so dramatically when they absorbed a UV photon. This led the astrochemists at NASA Ames and a competing group in France to investigate carbon-based soot particles. When the laboratory spectra produced such a good match to the UIR spectrum, both groups published their discoveries simultaneously. These soot particles are commonly described as PAH molecules.
The table below shows how the various bends and stretches in atomic bonds in the PAH molecule produce the UIR bands, according to the experts. The strongest bands are in bold.
| Wavelength (µm) | Assignment |
|---|---|
| 3.29 | Aromatic C-H stretch (v=1-0) |
| 3.40 | Aliphatic C-H stretch (v=1-0) (anti-symmetric mode) |
| 3.51 | Aliphatic C-H stretch (v=1-0) (symmetric mode) |
| 6.2 | C-C skeletal deformation |
| 7.7-7.9 | C-C skeletal deformations |
| 8.6 | C-H in-plane bend |
| 11.2 | C-H out-of-plane bend (solo mode) |
| 11.9 | C-H out-of-plane bend (duo mode) |
| 12.7 | C-H out-of-plane bend (trio mode) |
Our work in the 1990s considerably strengthened the case for the assignments given above to the 3.40 and 3.51 µm features.
Last modified 9 December, 2014. © Gregory C. Sloan.